Executive Summary
Safety Intelligence reports provide insights into safety of biologics and therapeutics drug products.
Enforcement Reports provides information on adverse event and medication error reports. The data is based on cases reported to FDA and supports post-marketing safety surveillance for drug and therapeutic biologic products. Medical Dictionary for Regulatory Activities (MedDRA) codes compliment the Adverse events and medication errors reported to FDA.
FAERS data includes the reports submitted to FDA by healthcare professionals - physicians, pharmacists, nurses and others) and consumers - patients, family members, lawyers and others. Adverse Event Reporting Intelligence reports are based on ten years of AERS data.
Types of Enforcement Reports - Class I, Class II & Class III
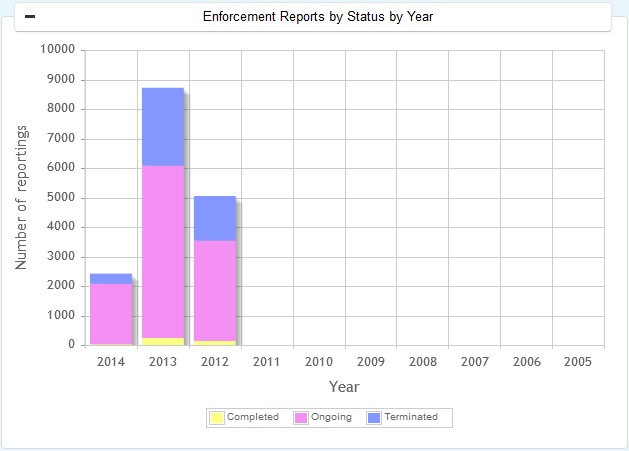
Recall is a action taken by a firm to remove a product from the market. Recalls are made by manufacturer's own initiative or by FDA, as statutory authority.
All the recalls classified as Class I Class II and Class III as well as unclassified recalls can be found in FDA Enforcement Reports. The product areas considered for reporting include - Drugs, Biologics, Medical Devices, Food, Cosemetics and Veterinary products.
FDA Enforcement Reports
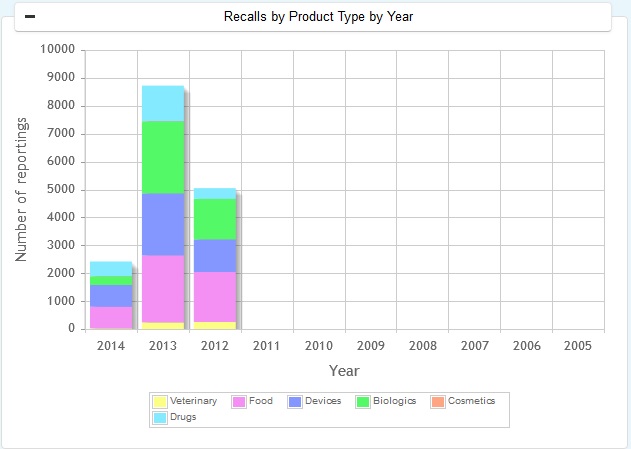
The product types for reporting include:
-
Drugs
-
Biologics
-
Medical Devices
-
Food
-
Cosemetics
-
Veterinary products


