Executive Summary
Generic drug manufacturing companies in Pharmaceutical Industry are constantly in pursuit of FDA approved brand names and generic drugs. It involves finding the generic drug products for a brand name product, finding drugs with specific ingredient as well as viewing the sponsored applicant and approval history of a drug. Another challenge encountered, is the lack of timely and up-to-date information on generic drugs. Most IT departments are not well equipped to incorporate regular FDA data updates. They also lack the resources needed to monitor FDA data releases.
VajraSoft Inc. PharmAnalytics TM is the Vertical Cloud Solution for Pharmaceutical Vertical Industry. In few clicks, it provisions FDA Approved Drug Products with Therapeutic Equivalence Evaluations as well as detailed product analytics including - Application details, Active ingredients, formulation, drug class, patent exclusivity, dosage and delivery methods.
Key Business Challenges
Key Business Challenges include:
-
Lack of timely and up-to-date consumer information on generic drugs
-
Continuously Monitor FDA data releases
-
Constantly perform database updates to get latest data
-
Maintain and support related database and front end
-
Incur costs for performing regular maintenance and support
Why PharmAnalytics?
VajraSoft Inc. – PharmAnalyticsTM delivers FDA Approved Drug Products with Therapeutic Equivalence Evaluations (FDA Orange Book & more).
VajraSoft Inc. – PharmAnalyticsTM provides the right information at right time. Analytics Dashboards and Reports of FDA Approved Drug Products with Therapeutic Equivalence Evaluations provide extensive data with drill down capabilities – providing valid product, application details, history of drug approvals and related changes – providing deep insights into drug information.
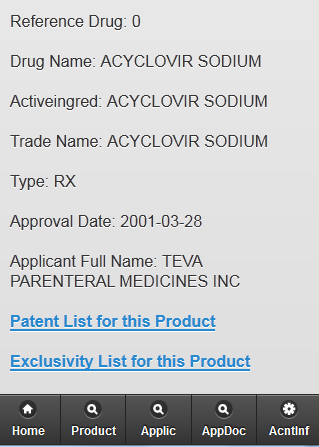
Using PharmAnalyticsTM access Reports of FDA approved drug products - both prescription drugs and over the counter (OTC) drugs as well as Discontinued drugs.
Key Features
-
PharmAnalyticsTM – Provide Deep insight into Patent, Product Active Ingredients – Make informed decisions.
-
Patent Information – extensive patent and market exclusivity information for FDA-approved drugs
-
Combines information from multiple sources
-
Full Exclusivity Code and Use Code descriptions
-
Analytics Dashboards and Reports of FDA Approved Drug Products with Therapeutic Equivalence Evaluations
-
Generate Reports, Search on Active Ingredients, Application Number, Patent number, Proprietary Name or Trade Name etc.
-
Prescription (Rx) and Over The Counter (OTC) Products list and cross references to applications
-
Reports on Active Ingredients
Benefits
Key benefits of PharmAnalyticsTM include:
-
Provide Timely information on FDA approved generic drugs
-
Analytics on-the-go, to make informed decisions
-
Regular updates to Data warehouse, providing requisite information on new generic drugs
-
Search by Active Ingredient, Proprietary name, Applicant, or Application Number
-
Provide Executives with deep insights into all FDA Approved drugs
-
Reduce the costs and increases efficiency


